MASUMOTO LAB
Overview
With the aging of society, the prevalence of cardiovascular diseases, including heart and vascular conditions, is expected to rise significantly. Meanwhile, heart transplantation—the ultimate treatment for severe cases—remains an extremely limited option. Therefore, developing new therapeutic approaches is essential to saving patients with advanced heart disease. Our laboratory is dedicated to pioneering next-generation medical treatments using iPS cells, leveraging the principal investigator’s clinical experience as a cardiovascular surgeon. We employ expertise in creating and treating surgical models of cardiovascular disease in animals to drive our research forward. In particular, we focus on the maturation of "cardiac organoids"—structures derived from iPS cells that replicate the architecture and function of the heart. Through this work, we aim to develop novel regenerative therapies for severe heart disease and advance drug discovery by recreating cardiac pathologies in vitro. To translate our research into clinical applications, we actively collaborate with various research institutions and industry partners, striving to bring innovative treatments to patients.
Our research
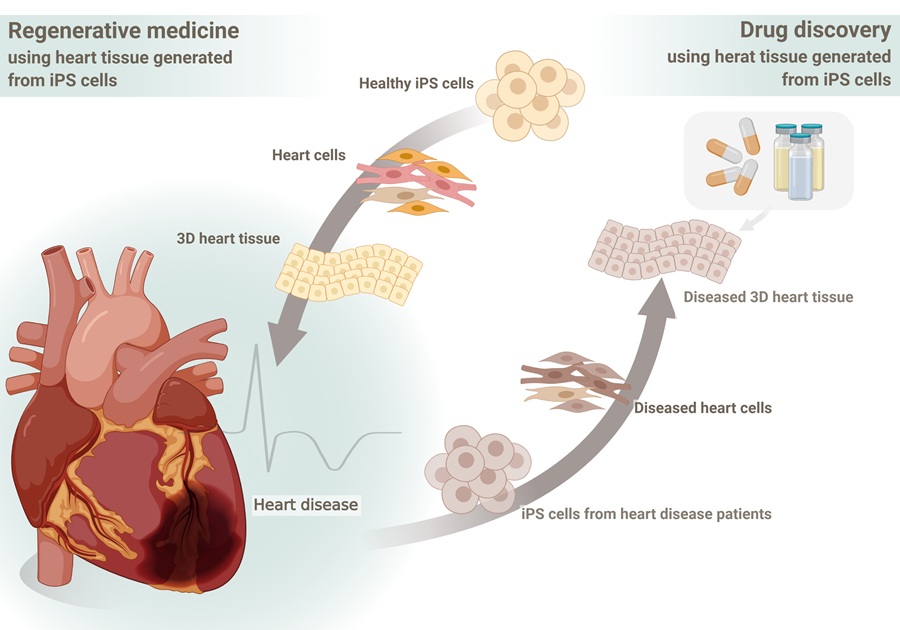
Regenerative Medicine
Our laboratory conducts a wide range of research in the field of regenerative medicine, with a particular focus on developing treatments for heart disease using iPS cells. In this field, we utilize various cardiac cell types, including cardiomyocytes differentiated from iPS cells, to explore new therapeutic approaches for conditions such as myocardial infarction and heart failure. In particular, our lab actively employs organoid technology based on tissue engineering approaches using iPS cells. We generate organoids that mimic the structure and function of the heart, aiming to elucidate the mechanisms of cardiac regeneration and achieve functional recovery of myocardial tissue—paving the way for "true" regenerative medicine. Additionally, we are investigating the application of biomaterials to enhance the therapeutic effects of regenerative medicine. These studies are expected to contribute to the future development of cardiac regenerative therapies.
(Reference)
Kuroda Y, Iida J, Murata K, Hori Y, Kobiki J, Minatoya K, Masumoto H*. Transplantation of vascularized cardiac microtissue from human iPS cells improves impaired electrical conduction in a porcine myocardial injury model. JTCVS Open. In press.
Iida J, Kotani K, Murata K, Hakamada K, Maihemuti W, Mandai Y, Hiraoka Y, Minatoya K, Masumoto H*. Retention of locally injected human iPS cell-derived cardiomyocytes into the myocardium using hydrolyzed gelatin. Sci Rep. 2025;15:4635. doi: 10.1038/s41598-025-87885-w.
Heima D, Takeda M, Tabata Y, Minatoya K, Yamashita JK*, Masumoto H*. Therapeutic potential of human induced pluripotent stem cell-derived cardiac tissue in an ischemic model with unloaded condition mimicking left ventricular assist device. J Thorac Cardiovasc Surg. 2024;168:e72-e88. doi: 10.1016/j.jtcvs.2023.11.019.
Osada H, Kawatou M, Fujita D, Tabata Y, Minatoya K, Yamashita JK*, Masumoto H*. Therapeutic potential of clinical-grade human induced pluripotent stem cell-derived cardiac tissues. JTCVS Open. 2021;8:359-374. doi: 10.1016/j.xjon.2021.09.038.
Ishigami M, Masumoto H*, Ikuno T, Aoki T, Kawatou M, Minakata K, Ikeda T, Sakata R, Yamashita JK*, Minatoya K. Human iPS cell-derived cardiac tissue sheets for functional restoration of infarcted porcine hearts. PLoS One. 2018;13:e0201650. doi: 10.1371/journal.pone.0201650.
Nakane T, Masumoto H, Tinney JP, Yuan F, Kowalski WJ, Ye F, LeBlanc AJ, Sakata R, Yamashita JK, Keller BB*. Impact of Cell Composition and Geometry on Human Induced Pluripotent Stem Cells-Derived Engineered Cardiac Tissue. Sci Rep. 2017;7:45641. doi: 10.1038/srep45641.
Masumoto H, Nakane T, Tinney JP, Yuan F, Ye F, Kowalski WJ, Minakata K, Sakata R, Yamashita JK, Keller BB*. The myocardial regenerative potential of three-dimensional engineered cardiac tissues composed of multiple human iPS cell-derived cardiovascular cell lineages. Sci Rep. 2016;6:29933. doi: 10.1038/srep29933.
Disease Modeling and Drug Discovery Research
Our laboratory utilizes iPS cells and organoid technology to model various diseases and explore potential drug discovery applications. In particular, we focus on developing disease models related to cardiovascular diseases and vascular disorders to better understand their pathophysiology and develop novel therapeutic strategies. We are also advancing drug discovery research using Organ-on-a-Chip (OoC) technology. By creating heart-on-a-chip models that mimic the structure of the heart and blood vessels, we can observe cellular interactions and drug responses under disease conditions. This technology serves as a promising alternative to animal experiments, facilitating the development of more human-relevant treatments. Through our disease modeling and drug discovery research, we aim to uncover disease mechanisms and develop innovative therapies, making significant contributions to both regenerative medicine and pharmaceutical advancements.
(Reference)
Murata K, Makino A, Tomonaga K*, Masumoto H*. Predicted risk of heart failure pandemic due to persistent SARS-CoV-2 infection using a three-dimensional cardiac model. iScience. 2023;27:108641. doi: 10.1016/j.isci.2023.108641. (※Cover image)
Abulaiti M, Yalikun Y, Murata K, Sato A, Sami MM, Sasaki Y, Fujiwara Y, Minatoya K, Shiba Y, Tanaka Y, Masumoto H*. Establishment of a heart-on-a-chip microdevice based on human iPS cells for the evaluation of human heart tissue function. Sci Rep. 2020;10:19201. doi: 10.1038/s41598-020-76062-w.
Kawatou M, Masumoto H, Fukushima H, Morinaga G, Sakata R, Ashihara T, Yamashita JK*. Modelling Torsade de Pointes arrhythmias in vitro in 3D human iPS cell-engineered heart tissue. Nat Commun. 2017;8:1078. doi: 10.1038/s41467-017-01125-y.

A study demonstrating the potential risk of heart failure due to persistent SARS-CoV-2 infection in the heart using human iPS cell-derived cardiac organoids (Murata,iScience2023), featured as the cover image of the February 2024 issue (Volume 27, Issue 2). ©Hayanon Science Manga Studio (2024)
Organoid Maturation
Our laboratory aims to enhance the maturation of organoids derived from iPS cells, bringing them closer to physiological conditions. Organoids are three-dimensional cellular aggregates that self-organize to mimic the structure of specific organs or tissues. However, achieving sufficient maturation remains a key challenge for improving the accuracy of regenerative medicine applications and disease modeling. To promote organoid maturation, we are exploring "physical training" approaches, including mechanical and electrical stimulation. In particular, for cardiac organoids, we apply mechanical loading to encourage contractile activity, facilitating the functional maturation of cardiomyocytes. These stimuli enhance the contractile force and electrophysiological activity of cardiac organoids, making them more physiologically relevant. Additionally, we are investigating the molecular mechanisms involved in organoid maturation and developing strategies to regulate these processes. For instance, activating specific transcription factors and cell signaling pathways has been explored to promote cardiomyocyte maturation. Improving cell-cell interactions within organoids is another approach to enhancing their overall maturation. To assess the functionality of matured organoids, we evaluate key parameters such as contractile force, electrophysiological activity, and metabolic function in cardiac organoids. Based on these assessments, we aim to identify the most effective stimuli and conditions to maximize the physiological functionality of organoids. Our research on organoid maturation is expected to significantly contribute to improving the precision of regenerative therapies and the reproducibility of disease models. Ultimately, it paves the way for more practical clinical applications in regenerative medicine.
(Reference)
Maihemuti W, Murata K, Abulaiti M, Minatoya K, Masumoto H*. Simultaneous electro-dynamic stimulation accelerates maturation of engineered cardiac tissues generated by human iPS cells. Biochem Biophys Res Commun. 2024;733:150605. doi: 10.1016/j.bbrc.2024.150605.
Message from Principal Investigator

Project-Specific Professor: Hidetoshi MASUMOTO, MD, PhD
masumoto [at] kuhp.kyoto-u.ac.jp
Dr. Masumoto earned his MD from Kyoto University in 1999. After serving as a cardiovascular surgeon at Kumamoto Central Hospital and Shizuoka City Shizuoka Hospital, he pursued research as a postdoctoral fellow at the University of Louisville, USA. He later held positions as a specially appointed assistant professor at the Center for iPS Cell Research and Application (CiRA), Kyoto University. In 2023, he was appointed as a Project-Specific Associate Professor in the Department of Cardiovascular Surgery at Kyoto University Hospital, and he assumed his current position in April 2025. From October 2017 to March 2025, he led a research group as a ResearchLeader in the Clinical Translational Research Program at the RIKEN Center for Biosystems Dynamics Research. From April 2025, he continues his affiliation with RIKEN as a Visiting Principal Investigator. In 2023, he became the first researcher in Japan to be awarded the prestigious NIBR Global Scholars Program grant from Novartis Institutes for BioMedical Research. His research focuses on the structural and functional maturation of cardiac organoids derived from iPS cells, aiming to develop novel regenerative therapies for severe heart diseases and advance drug discovery through disease modeling.
Pioneering the Future of Regenerative Medicine—Towards the Innovation of Next-Generation Cardiovascular Therapy
Cardiovascular disease is becoming an increasingly critical issue in aging societies. However, treatment options for severe heart disease remain limited, highlighting the urgent need for new therapeutic strategies. Our research focuses on constructing and functionally maturing cardiac organoids derived from iPS cells, aiming to establish novel regenerative therapies for heart disease. By doing so, we seek to provide treatment options that do not rely on transplantation and accelerate drug discovery through advanced disease modeling. This project is deeply committed to bridging the gap between basic research and clinical application. We collaborate with experts across multiple disciplines, including medicine, engineering, pharmaceuticals, and biotechnology, to develop and implement next-generation cardiovascular therapies. In particular, we are driving the development of innovative treatment strategies utilizing cell technologies and biomaterials, as well as creating frameworks to bring these advancements to clinical practice. Such an ambitious challenge requires the collective efforts of researchers, physicians, and industry partners. By integrating diverse fields and leveraging each other's strengths, we believe groundbreaking therapies can emerge. We are dedicated to shaping the future of cardiovascular medicine and delivering new hope to patients. Together, let us continue this journey toward innovation and better healthcare.
Selected Publications
- Kuroda Y, Iida J, Murata K, Hori Y, Kobiki J, Minatoya K, Masumoto H*. Transplantation of vascularized cardiac microtissue from human iPS cells improves impaired electrical conduction in a porcine myocardial injury model. JTCVS Open. In press. doi: doi.org/10.1016/j.xjon.2025.03.006.
- Murata K, Makino A, Tomonaga K*, Masumoto H*. Predicted risk of heart failure pandemic due to persistent SARS-CoV-2 infection using a three-dimensional cardiac model. iScience. 2023;27:108641. doi: 10.1016/j.isci.2023.108641. (※Cover image)
- Kyo S, Murata K, Kawatou M, Minatoya K, Sunagawa GA*, Masumoto H*. Quiescence-inducing neurons-induced hypometabolism ameliorates acute kidney injury in a mouse model mimicking cardiovascular surgery requiring circulatory arrest. JTCVS Open. 2022 Nov 8;12:201-210. doi: 10.1016/j.xjon.2022.11.001.
- Abulaiti M, Yalikun Y, Murata K, Sato A, Sami MM, Sasaki Y, Fujiwara Y, Minatoya K, Shiba Y, Tanaka Y, Masumoto H*. Establishment of a heart-on-a-chip microdevice based on human iPS cells for the evaluation of human heart tissue function. Sci Rep. 2020;10:19201. doi: 10.1038/s41598-020-76062-w.
- Kawatou M, Masumoto H, Fukushima H, Morinaga G, Sakata R, Ashihara T, Yamashita JK*. Modelling Torsade de Pointes arrhythmias in vitro in 3D human iPS cell-engineered heart tissue. Nat Commun. 2017;8:1078. doi: 10.1038/s41467-017-01125-y.
- Setozaki S, Minakata K*, Masumoto H, Hirao S, Yamazaki K, Kuwahara K, Ikeda T, Sakata R. Prevention of abdominal aortic aneurysm progression by oral administration of green tea polyphenol in a rat model.
J Vasc Surg. 2017;65:1803-1812.e2.
doi: 10.1016/j.jvs.2016.06.003.
- Masumoto H, Ikuno T, Takeda M, Fukushima H, Marui A, Katayama S, Shimizu T, Ikeda T, Okano T, Sakata R, Yamashita JK*. Human iPS cell-engineered cardiac tissue sheets with cardiomyocytes and vascular cells for cardiac regeneration. Sci Rep. 2014;4:6716. doi: 10.1038/srep06716.
- Masumoto H, Matsuo T, Yamamizu K, Uosaki H, Narazaki G, Katayama S, Marui A, Shimizu T, Ikeda T, Okano T, Sakata R, Yamashita JK*. Pluripotent stem cell-engineered cell sheets reassembled with defined cardiovascular populations ameliorate reduction in infarct heart function through cardiomyocyte-mediated neovascularization. Stem Cells. 2012;30:1196-205. doi: 10.1002/stem.1089.



